Key findings
Introduction
Optical Genome Mapping (OGM) is a is a novel high-throughput diagnostic technique that overcomes the limitations of conventional cytogenetic methods. The objective of this study was to evaluate the application of OGM in the daily workflow of the cytogenetics laboratory and compare it with standard techniques.
Methods
A total of 180 adult or pediatric patients referred to the Cytogenetics Laboratory at Hospital La Fe during disease onset or relapse were included. The patients had the following diagnoses: aplastic anemia (AA) n = 6, B-cell acute lymphoblastic leukemia (B-ALL) n = 39, T-cell acute lymphoblastic leukemia (T-ALL) n = 9, acute myeloid leukemia (AML) n = 51, myelofibrosis (MF) n = 36, polycythemia vera (PV) n = 3, essential thrombocythemia (ET) n = 2, multiple myeloma (MM) n = 11, and myelodysplastic syndromes (MDS) n = 22. Chromosome banding analysis (CBA) was performed on bone marrow samples, and in selected cases, fluorescence in situ hybridization (FISH) was carried out using specific probes following diagnostic recommendations. For OGM analysis, high molecular weight DNA was extracted and labeled from peripheral blood (PB, n = 62) or bone marrow (BM, n = 118) following the manufacturer's protocol (Bionano, San Diego, CA, USA).
Results
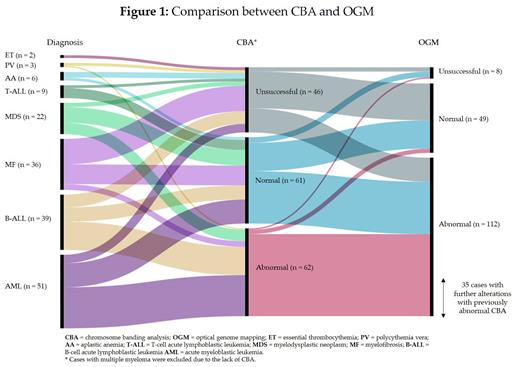
CBA was performed on all patients except MM, where an initial FISH panel targeting IGH rearrangement, TP53 deletion, and 1q amplification was applied. Among the CBA, 61/169 (36%) patients showed a CBA without alterations, 62/169 (37%) were abnormal and 46/169 (27%) were unsuccessful. OGM successfully detected cryptic alterations in 60% (37/61) of cases previously classified as normal with CBA. Additionally, OGM identified further alterations in 56% (35/62) of cases with abnormal CBA findings. Notably, OGM demonstrated a remarkable 93% success rate in resolving previously uninformative CBA results.
AA OGM did not detect clinically significant alterations or copy neutral loss of heterozygozity (CN-LOH) on chromosome 6 in this subgroup of patients.
B-ALL: OGM detected alterations in all patients with a normal or uninformative karyotype. Regarding cases with a previous abnormal CBA, OGM discovered 2 cases with masked hypodiploidy. Copy Number Alterations (CNA) affecting IKZF1, PAX5, ETV6, RB1, BTG1, EBF1, CDKN2A, CDKN2B, PAR1 region, and ERG were detected in 27/39 (69%) patients, 3 of whom harbored the IKZF1plus Moreover, several cryptic rearrangements were uncovered, such as MEF2D::BCL9, RUFY1::ETV6, PDGFRB::EBF1, and PAX5::PML.
T-ALL: All patients showed an uninformative or normal CBA. However, OGM discovered rearrangements in 7/9 (78%) patients, including PICALM::MLLT10, GATA3::RUNX1, or STIL::TAL1.
AML OGM enabled the detection of 4 cases with cryptic NUP98::NSD1 rearrangements and 2 cases with tandem duplication of KMT2A. Additionally, OGM successfully identified the partner involved in a previously uncharacterized KMT2A rearrangement ( KMT2A::ELL).
MPN (MF, PV and ET) = The main utility of OGM was observed in MF, as it resolved all cases with uninformative CBA.
MM Primary limitations in these cases were low plasma cell infiltration and difficulty in DNA extraction from total nucleated cells. Only one case showed an infiltration rate higher than 15%, as determined by flow cytometry. Consequently, 7 cases obtained a normal OGM result despite alterations detected by FISH. However, OGM successfully identified a cryptic rearrangement involving MYC and provided a detailed characterization of CNA in the remaining cases.
MDS OGM resolved uninformative karyotypes in the subgroup of MDS with fibrosis. Additionally, the resolution of OGM allowed the detection of a t(3;8)(q26.2;q24.21) with a variant allele frequency of 6%, involving MECOM rearrangement, in a patient who later progressed to AML.
In total, 8 cases were uninformative with OGM, mainly because of poor sample quality or low cellularity.
Conclusion
This study demonstrates the utility of integrating emerging technologies like OGM into the daily routine of the laboratory. DNA extraction should be performed through selected CD138 cells in multiple myeloma
Disclosures
De La Rubia:Janssen: Honoraria, Speakers Bureau; Pfizer: Speakers Bureau; Sanofi: Speakers Bureau; Takeda: Research Funding; Menarini: Honoraria; Oncopharm: Honoraria; GSK: Honoraria, Research Funding, Speakers Bureau; BMS: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal